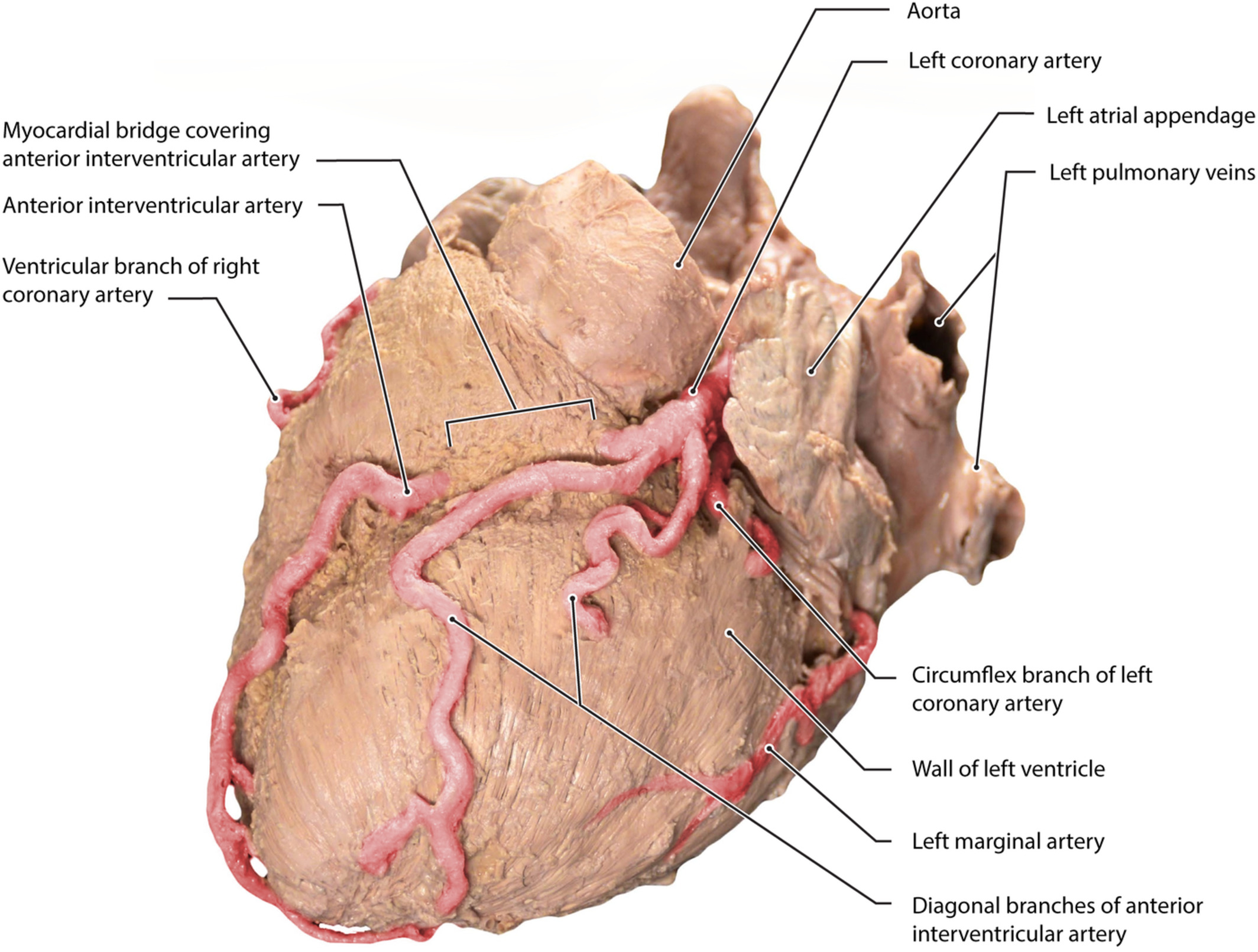
Surgical correction in re-do mitral valve surgery
Rheumatic heart disease (RHD) is the only preventable cardiovascular disease which causes significant morbidity and mortality particularly in low- and middle-income countries. The need for surgery for severe RHD is clear with a significant lack of access to surgical and percutaneous intervention in regions of the greatest global need. Surgery for RHD should be timeous to result in the best possible outcome. In advanced RHD, heart failure, atrial fibrillation and endocarditis can add to the complexity of the surgery.
History
A 45-year-old Sudanese lady, with history of mitral valve repair done 20 years prior, presented with dyspnea on exertion, chest pain and severe pulmonary artery hypertension. Echocardiogram showed severe mitral stenosis, moderate mitral regurgitation, moderate tricuspid regurgitation, severe pulmonary artery hypertension, enlarged right atrium and giant left atrium. Coronary angiogram showed normal coronaries.
Surgical Procedure
Under cardiopulmonary bypass and cardioplegic arrest, she underwent redo mitral valve replacement (29mm carbomedics standard mechanical valve), tricuspid valve repair with Annuloplasty (32mm Edwards MC3 Annuloplas-ty ring), left atrial appendage closure from left atrium, left atrial size reduction with plication, and right atrial size reduction. Came off CPB in the first attempt, in sinus rhythm. Patient made a gradual uneventful recovery post-op and was discharged home on the tenth post-operative day.
Discussion
RHD remains a neglected cardiovascular disease, which causes significant morbidity and mortality in low- and middle-income countries. Timing of surgical intervention is crucial for a favorable outcome. Our patient with history of previous mitral valve repair, presented relatively late with compounding risk factors of cardiac failure, atrial fibrillation, pulmonary artery hypertension, tricuspid regurgitation and giant left atrium, in addition to the primary pathology of mitral valve disease. Giant left atrium (GLA) is a condition defined when the left atrial diameter exceeds 65 mm. It is commonly associated with mitral valve regurgitation due to excess intracavitary pressure resulting in strain and dilation of the left atrial chamber. The enlarged left atrium leads to expansion of left atrial volume, which in turn can place pressure on the main bronchus, lung and left ventricle with corresponding cardiopulmonary embarrassment. Because GLA can increase the risk of sudden death, its existence merits surgical intervention. Partial resection of inferior and or superior left atrial wall, is the most common surgical technique. With the evolution of atrial fibrillation surgery, atrial size matters and is determinant of long term performance following successful ablation. Surgical management of GLA achieves good clinical outcome with respect to cardiopulmonary performance including restoration of sinus rhythm among patients suffering from atrial fibrillation.
In patients who undergo mitral valve surgery with tricuspid regurgitation or tricuspid annular dilation, performing concomitant tricuspid valve repair lowered risk for progression of tricuspid regurgitation. There is broad agreement that when a patient has severe tricuspid regurgitation, then that valve should be repaired. However, there is significant uncertainty in how to manage moderate or less tricuspid regurgitation. There are some data from observational studies that failure to manage less than severe tricuspid regurgitation is associated with decreased survival and heart failure. It is a common debate among surgical team members as to what to do with leaking tricuspid valve during mitral valve surgery. It is recommended that the tricuspid valve annulus be intervened based on function (i.e., degree of tricuspid valve regurgitation), diameter size, significant right ventricular dilation or dysfunction. This patient presented all the difficulties associated with rheumatic valve surgery including cardiac failure, atrial fibrillation, associated valve lesion, pulmonary artery hypertension and giant left atrium. Careful assessment, pre-operative optimization, meticulous surgical technique and optimal post-operative management played an important role in successful outcome.